Periodic table and the electrons
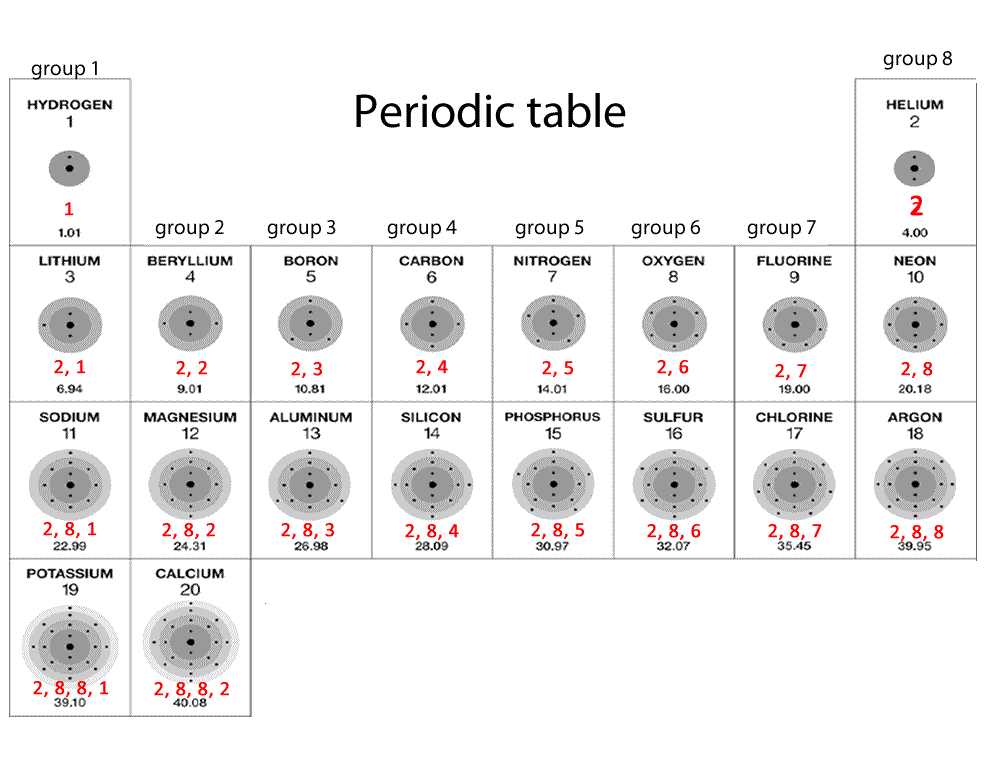
The modern periodic table has elements arranged in order of electronic configuration. The table is divided into groups(columns) and periods(rows). Elements in the same group display similar chemical properties and trends immerge as we go along a period and down a group.
Click to see how size of atoms changes across a period and down a group.
Look at the electronic configuration of each atom. Can you explain the trend in atomic radius?
All elements in a particular group share similar properties. All elements in group 1 are very reactive metals, with the exception of hydrogen gas. These metals react readily with water to produce hydrogen gas. Click on the blue writing below to see the reactions.
Elements in group 2 also react with water to form hydrogen gas but are not as reactive as group 1 elements.
Elements in group 8 exist as gases and are very stable. These are known as noble gases and do not combine with other atoms. Hence, they are always monoatomic.
Elements in group 4, 5, 6,7 and 8 are nonmetals.
Elements in group 1 have ONE electron in their outer most energy level. Similarly atoms in groups 2-8 have 2,3,4,5,6,7 or 8 electrons respectively.
An element in period 2 has the second energy level as the last level to be occupied by electrons. Elements in period 4 have the forth energy level as the outer most level to be occupied by electrons.
So an element found in period 2, group 2, namely magnesium, should have two electrons in the last energy level, which is level 2 as depicted by the period number; in this case it is period 2.
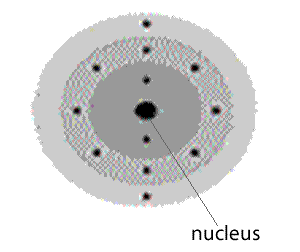
An atom with 12 electrons will be expected to look like the atom on the left. So we can expect to find this element in the period 3, group 2.
Complete the table below. The first one is done for you
| Element | Atomic number | Electronic configuration | Group | Period |
| Al | 13 | 2,8,3 | 3 | 3 |
| Mg | 12 | |||
| Na | 11 | |||
| F | 9 |
All elements in the same group
share similar properties.
a) What can you say about the elements in group 1.
b) How do the elements in group one differ from those in group 8.
c) An element with the atomic number 14 is found in group of the periodic table because it has . This element will be found in period of the periodic table because it has
d) Do all elements in group 8 have 8 valence electrons? Explain